d2 Trimer and d3 Tetramer in a Pyrochlore Lattice
© The Physical Society of Japan
This article is on
Charge Disproportionation for Trimerization and Tetramerization in Spinel-Type AlV2O4
(JPSJ Editors' Choice)
J. Phys. Soc. Jpn. 93, 074709 (2024).
Based on the charge disproportionation of V3+ and V2+, the V3+(d2) trimers and V2+(d3) tetramers in the vanadium pyrochlore lattice of AlV2O4 are described by the orbitally-induced Peierls mechanism.
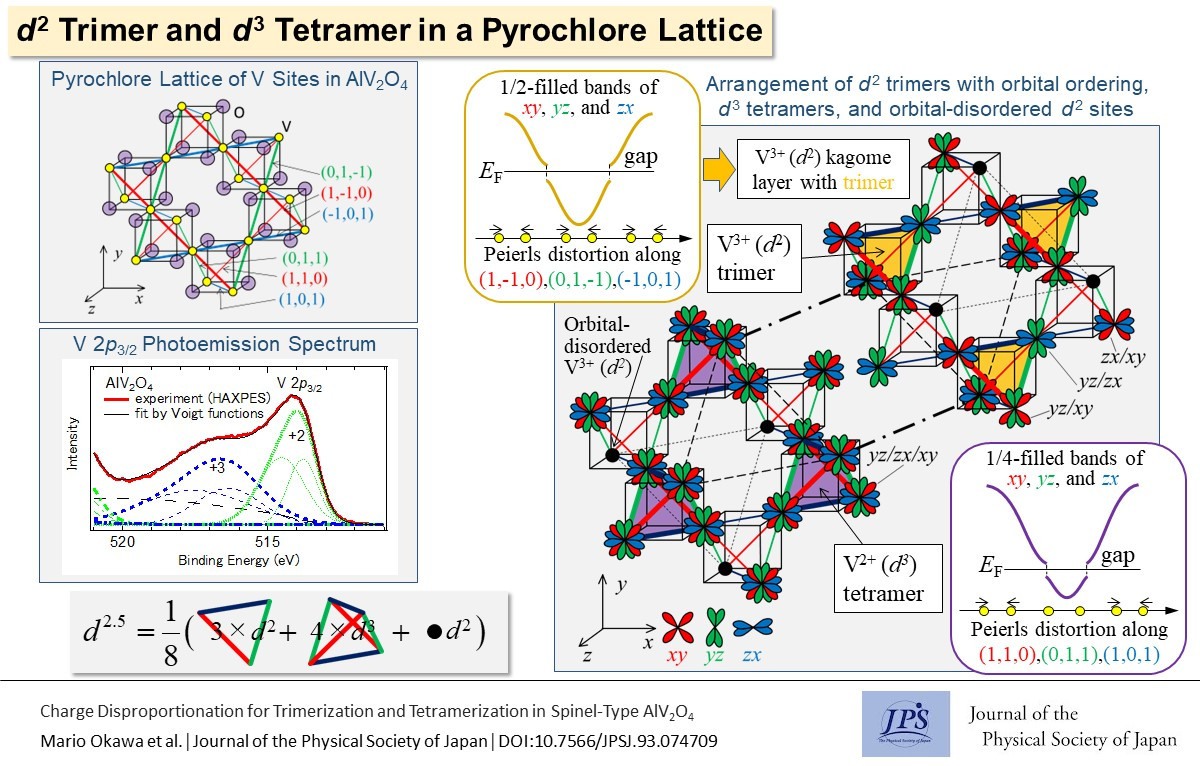
When a one-dimensional band is partially filled with electrons in a one-dimensional lattice, the lattice undergoes Peierls distortion with a new periodicity that can create a bandgap at the Fermi level. When the one-dimensional band is half(quarter)-filled, the Peierls distortion results in a two(four)-fold periodicity of the original lattice. Spinel-type transition metal compounds with partially filled xy, yz, or zx orbitals often exhibit lattice distortions that can be interpreted as Peierls distortions of one-dimensional xy bands along the (1,1,0) and (1,-1,0) chains, yz bands along the (0,1,1) and (0,1,-1) chains, or zx bands along the (1,0,1) and (1,0,-1) chains of the pyrochlore lattice formed by the transition metal sites. For example, in CuIr2S4, the Ir pyrochlore lattice undergoes octamerization because of Peierls distortion (Ir–Ir dimerization), and it displays a four-fold periodicity along the (1,1,0) and (1,-1,0) directions, which are selected by the Ir 5d orbital polarization (in which only the xy band is partially filled). This microscopic description of multimer formation is called the orbitally-induced Peierls mechanism.
In spinel-type AlV2O4, the vanadium pyrochlore lattice contains vanadium trimers and tetramers. It is difficult to describe this complicated distortion using the orbitally-induced Peierls mechanism. Using hard-X-ray photoemission spectroscopy (HAXPES) performed at SPring-8, Okawa et al. showed that V2+ and V3+ coexisted in a 1:1 ratio in AlV2O4. The main and shoulder peaks of the V 2p3/2 core-level HAXPES spectra were assigned to V2+ with a d3 configuration and V3+ with a d2 configuration, respectively. Based on the charge disproportionation of V2+:V3+=1:1, the formation of V trimers and tetramers in AlV2O4 is explained by an orbitally-induced Peierls mechanism, in which the half-filled xy, yz, and zx bands along the (1,-1,0), (0,1,-1), and (-1,0,1) chains yield V3+ trimers. The extra electrons of V2+ are accommodated in the xy, yz, and zx bands along the (1,1,0), (0,1,1), and (1,0,1) chains, thereby creating quarter-filled bands. The short V–V bonds were created by Peierls distortions with a four-fold periodicity and connected to the trimers. Half of the V3+(d2) trimers were transformed into V2+(d3) tetramers. This work suggests that the interaction between the charge and orbital degrees of freedom plays a vital role in the multimer formation of various transition metal compounds.
(Written by T. Mizokawa and T. Saitoh on behalf of all the authors)
Charge Disproportionation for Trimerization and Tetramerization in Spinel-Type AlV2O4
(JPSJ Editors' Choice)
J. Phys. Soc. Jpn. 93, 074709 (2024).
Share this topic
Fields
Related Articles
-
Pressure-Tuned Classical–Quantum Crossover in Magnetic Field-Induced Quantum Phase Transitions of a Triangular-Lattice Antiferromagnet
Magnetic properties in condensed matter
Electron states in condensed matter
Cross-disciplinary physics and related areas of science and technology
2024-9-5
The correspondence principle states that as quantum numbers approach infinity, the nature of a system described by quantum mechanics should match that described by classical mechanics. Quantum phenomena, such as quantum superposition and quantum correlation, generally become unobservable when a system approaches this regime. Conversely, as quantum numbers decrease, classical descriptions give way to observable quantum effects. The external approach to classical–quantum crossover has attracted research interest. This study aims to demonstrate a method for achieving such control in materials.
-
Discovery of Light-Induced Mirror Symmetry Breaking
Dielectric, optical, and other properties in condensed matter
Electronic transport in condensed matter
2024-9-2
The authors discovered the light-induced mirror symmetry breaking, paving the way for controlling mirror symmetries via light and for realizing various phenomena utilizing the mirror symmetry breaking.
-
Structural Rotation and Falsely Chiral Antiferromagnetism: A New Combination Generating Ferrotoroidic State
Magnetic properties in condensed matter
Dielectric, optical, and other properties in condensed matter
2024-7-4
The ferrotoroidic state, an exotic state of matter with broken space inversion and time-reversal symmetries, was achieved by combining structural rotation and falsely chiral antiferromagnetism in PbMn2Ni6Te3O18.
-
Evaluation of the Exchange Stiffness Constants of Itinerant Magnets from the First-Principles Calculations
Electron states in condensed matter
Structure and mechanical and thermal properties in condensed matter
2024-6-5
Using first-principles calculations, we evaluated the exchange stiffness constants of ferromagnetic metals at finite temperatures. The constants can be used as parameters in the Landau–Lifshitz–Gilbert equation.
-
Current Melt Frozen Electrons
Dielectric, optical, and other properties in condensed matter
Magnetic properties in condensed matter
2024-1-15
The origin of the current-induced insulator-to-metal transition of samarium monosulfide was explained by the 4f−5d hybridization observed using optical reflectivity and photoelectron spectroscopies.
