Descriptor of Thermogalvanic Cell for Waste-heat Harvesting
© The Physical Society of Japan
This article is on
Scaling Relation between Electrochemical Seebeck Coefficient for Fe2+/Fe3+ in Organic Solvent and Its Viscosity
(JPSJ Editors' Choice)
J. Phys. Soc. Jpn.
90,
033602
(2021)
.
This article is on
Waste Heat Harvesting: Descriptor of Thermogalvanic Celln
JPSJ News Comments
18,
7
(2021)
.
The scaling relation between the Seebeck coefficient and solvent viscosity would enable for the design of improved thermogalvanic cells.
Harvesting low-grade heat from industrial processes significantly improves energy efficiency in operating factory activities. Because converting thermal energy into electricity enables ceaseless access to clean energy, high-performance thermoelectric devices are an essential technology for sustainable development [1].
Thermogalvanic cells (or thermo-electrochemical cells), which are composed of a liquid electrolyte with hot and cold electrodes, are capable of efficiently converting thermal energy into electricity [2, 3]. However, a microscopic mechanism that dominates the performance of thermogalvanic cells is yet to be fully understood, hindering their performance improvement and widespread deployment.
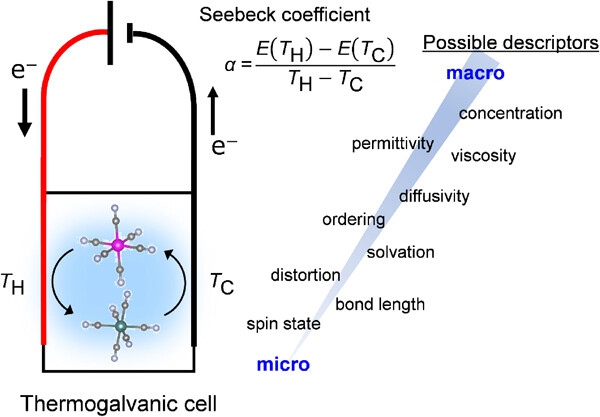
Fig. 1. Schematic illustration of a thermogalvanic cell. Determining the descriptors of the Seebeck coefficient of a redox couple is primarily important to achieve efficient waste-energy harvesting.
The research group led by Moritomo [4] identified the scaling relation between the Seebeck coefficient (α) of [FeIII(CN)6]3-/[FeII(CN)6]4-redox couple and the viscosity (η) of solvents, where α ranges from 0.14 mV/K (glycerin, η = 1412 mPa s) to 3.6 mV/K (acetone, η= 0.3 mPa s) as a function of η‒0.4. X-ray absorption near-edge structure (XANES) showed the deformation of Fe2+coordination environment with decreasing η, suggesting that a low-ηsolvent weakly interacts with a solute to provide a large configuration-entropy change upon redox, and thus large α.
To clarify the origin for this scaling relation, the microscopic understandings of both η and α are required. Large-scale quantum mechanics/molecular mechanics (QM/MM) calculation of electrolytes could provide accurate electrolyte structures, shedding light on the atomistic mechanism [5]. In addition, considering a relatively low coefficient of determination (R2) for the scaling relation, α is also affected by other macroscopic/microscopic descriptors. Following systematic data collection, state-of-the-art machine learning analyses could determine discriminating features [6], and the resulting accurate performance prediction could enable the development of advanced thermogalvanic cells for sustainability.
References
[1] J. He and T. M. Tritt, Science 357, eaak9997 (2017).
[2] T. I. Quickenden and Y. Mua, J. Electrochem. Soc. 142, 3985 (1995).
[3] H. J. V. Tyrrell, D. A. Taylor, and C. M. Williams, Nature 177, 668 (1956).
[4] D. Inoue, H. Niwa, H. Nitani, and Y. Moritomo, J. Phys. Soc. Jpn. 90, 033602 (2021).
[5] Y. Tateyama, J. Blumberger, M. Sprik, and I. Tavernelli, J. Chem. Phys. 122, 234505 (2005).
[6] A. Chen, X. Zhang, and Z. Zhou, InfoMat 2, 553 (2020).
Scaling Relation between Electrochemical Seebeck Coefficient for Fe2+/Fe3+ in Organic Solvent and Its Viscosity
(JPSJ Editors' Choice)
J. Phys. Soc. Jpn.
90,
033602
(2021)
.
Waste Heat Harvesting: Descriptor of Thermogalvanic Celln
JPSJ News Comments
18,
7
(2021)
.





