Toward a Hydrogen Society: Harnessing the Power of the Smallest Element
© The Physical Society of Japan
This article is on
JPSJ Special Topics on "Frontier of Hydrogen Science"
J. Phys. Soc. Jpn. 89, No. 5 (2020).
Comprehensive curated reviews by researchers on the latest research in hydrogen-related applications, including proton conductors, superconductivity, electrochemistry with hydrides, and more.
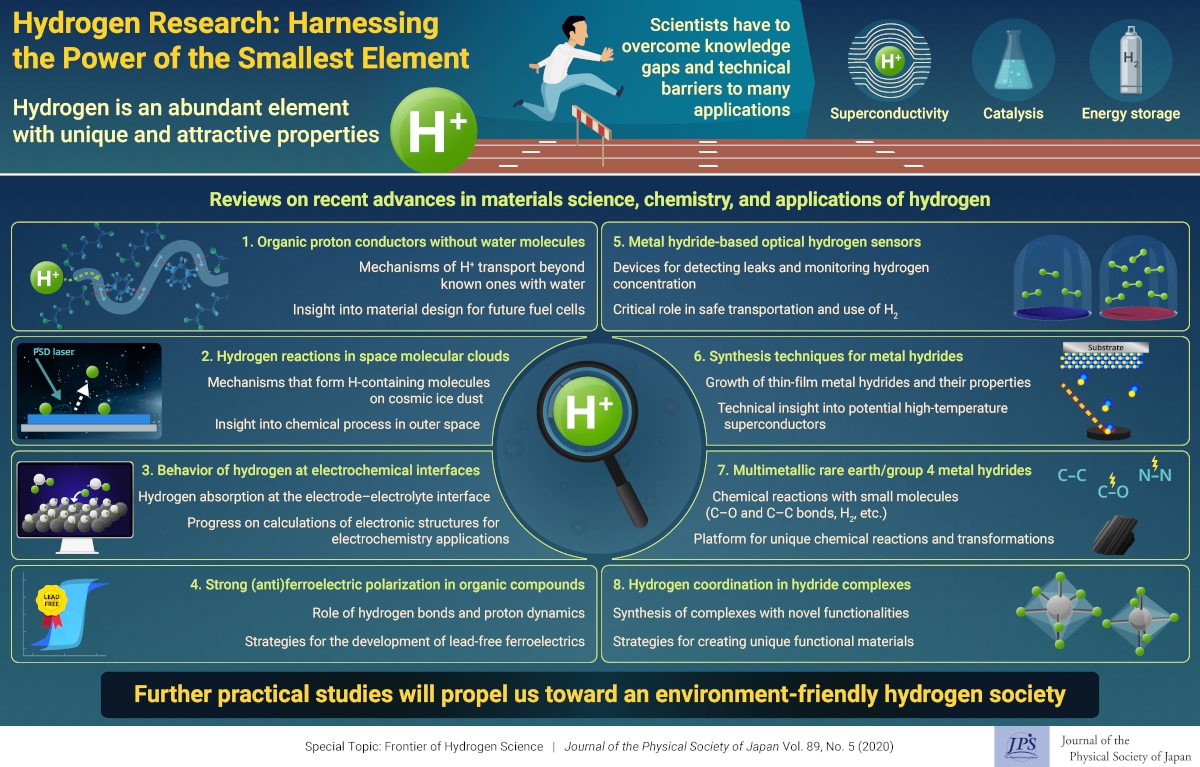
For such a seemingly simple element, hydrogen has a surprisingly huge number of unique properties that have attracted the attention of many scientists worldwide. Aside from its many useful electrochemical characteristics, hydrogen is on its way to become tomorrow’s preferred environment-friendly fuel; it may be our best bet in the constant struggle to protect the planet from ourselves.
Though hydrogen science is currently at its peak, there are still many hurdles to overcome before we can tap into hydrogen’s full potential. To put current and future researchers up to date with the latest advances related to hydrogen, this special issue of the Journal of the Physical Society of Japan includes a Special Topics section containing several review papers on different themes in hydrogen science. These papers cover both fundamental questions related to hydrogen behavior and progress in applications involving hydrogen and hydrogen ions.
For example, hydrogen-containing materials could become useful superconductors. Superconductivity at room temperature is highly sought after because of its vast number of potential applications, from lossless energy transport to quantum computing. Thin-film metal hydrides, though difficult to synthesize, may be the key to achieving this feat. One of the review papers focuses on available techniques to produce these materials and an overview of their interesting characteristics.
What about the power of hydrogen as chemical tool? Metal hydrides, which contain hydrogen in its negative ion state (H−), can catalyze a variety of useful chemical reactions and cleave many types of bonds, even in small molecules. One review paper focuses on recent discoveries on the synthesis of multimetallic metal hydrides, particularly those containing rare-earth metals or group 4 transition metals. A more thorough understanding of these peculiar materials would let us harness them as efficient platforms for tailored chemical reactions and transformations.
Another promising application of metal hydrides is in fast optical hydrogen sensors, which are critically important given that hydrogen gas is highly explosive. Thus, one review paper is dedicated to recent progress in the development of color-changing hydrogen sensors. The ability to detect hydrogen leaks will come in handy as we adopt hydrogen as the clean fuel powering tomorrow.
However, to make a hydrogen society a reality, safe and efficient fuel cells are a requirement. In this regard, organic water-free materials with high H+ conductivity could be an attractive design option for future fuel cells. One review paper explores our understanding of proton conduction and its underlying mechanisms, derived by using custom designed organic structures.
Many upcoming electrochemistry and electronics applications involving hydrogen rely on having a firm grasp on the effects of hydrogen in materials and how hydrogen atoms interact with each other and with metals centers. One paper focuses on this topic, referred to as ‘hydrogen coordination,’ in a variety of hydride complexes with many hydrogen atoms. Further progress in this area would allow us to create unique functional materials. Meanwhile, another review paper delves into the role of hydrogen and hydrogen ions in organic materials with strong magnetic properties. It summarizes current techniques for synthesizing and assessing the ferroelectric characteristics of organic compounds. Understanding hydrogen-related phenomena in such materials will bolster the development of lead-free ferroelectric devices.
In line with modern computing capabilities, simulations and calculations based on first principles have become increasingly common. Unfortunately, as highlighted in one of the review papers, there are no entirely convincing first-principle methods for understanding what happens with hydrogen at the electrode–electrolyte interface. The authors review the associated theory and explain how typical calculations are not enough to describe even this relatively simple system. They propose refinements to conventional methods that will help obtain better results to match experimental data.
Finally, the last review paper goes beyond the earthly uses of hydrogen and instead focuses on the chemical evolution of the universe. The authors focus on understanding how hydrogen behaves on the surface of cosmic ice dust, where it is believed to play a crucial role in the formation of molecules in interstellar clouds, the birthplace of stars and planets. To this end, they review their recently proposed techniques for detecting hydrogen on the surface of various types of ice samples, used as substitutes for cosmic dust in experiments on Earth.
It is clear from these reviews that hydrogen will take the spotlight in countless applications in the near future. Understanding its complex role in materials will let us take advantage of its full potential, propelling us toward a much-needed hydrogen society.
JPSJ Special Topics on "Frontier of Hydrogen Science"
J. Phys. Soc. Jpn. 89, No. 5 (2020).
